Essure sterilization, a method of permanent contraception that employs a “high-risk” medical device, has reportedly harmed thousands of women throughout the country. Though the manufacturer, Bayer, advertises Essure as “worry-free” birth control, over 10,000 injured patients have filed complaints to the FDA, saying that the device causes a host of serious side effects and complications.
Victims commonly describe suffering from “excruciating” pain, organ damage, severe metal allergy symptoms, and some have even developed chronic autoimmune diseases after being implanted with Essure.
In response to these widespread complaints, the FDA conducted a thorough investigation of Essure starting in September 2015, then announced plans to update Essure labeling with an urgent “black box” safety warning in March 2016. However, many injured patients, concerned medical professionals, and healthcare advocates consider the proposed label change a sorely inadequate measure for protecting women from the dangers of Essure. Many critics continue to rally support in the fight for a complete ban of the device.
Bayer now faces lawsuits from hundreds of women who say the manufacturer misled them by advertising Essure as “safe and effective,” causing them to suffer severe injuries that they believe could have been avoided with a safer form of permanent birth control.
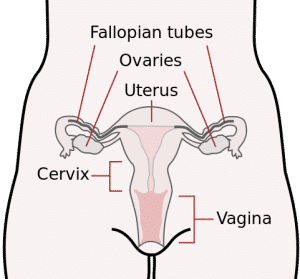
Essure is an implantable metal device designed to sterilize women by closing off the fallopian tubes. Unlike the more widely-used tubal ligation method, a reversible procedure in which the fallopian tubes are clamped or burned (cauterized) shut, Essure causes the body to form a permanent barrier of scar tissue. This barrier stops sperm from reaching the eggs, inhibiting conception.
Though Essure is currently manufactured by Bayer, a leading multinational healthcare company headquartered in Germany, the device was originally developed by Conceptus, Inc., a California-based corporation that became a Bayer subsidiary in June 2013. Essure was approved by the FDA in 2002.
Find more articles from Banville Law, here.

The Essure system is made of two small metal coiled “micro-insert” implants. Each micro-insert has a nickel-titanium outer coil and a stainless steel inner coil covered in polyethylene terephthalate (PET) fibers, more commonly known as polyester.
The implants are designed to be placed simultaneously, one in each fallopian tube, by means of a delivery catheter. The catheter is inserted into the woman’s body through the vagina and used to position the implants into the fallopian tubes, right where they open up into the uterus. The implantation procedure is monitored using a hysteroscope, a long, lighted tool equipped with a camera.
Once correct placement is achieved, the physician releases the implants and removes the catheter. Upon release, the implants expand so that the flexible outer coils press tightly against the walls of the fallopian tubes, adjusting to their unique geometry and “locking in” the device’s position. According to Bayer, this implantation procedure takes less than an hour to complete and generally requires no downtime for recovery.
Bayer promotes Essure sterilization as “surgery-free,” but the implantation procedure is classified as surgery by hospitals, medical boards, and insurance companies. According to medical experts who spoke at a 2015 FDA panel meeting on Essure, though the device’s placement doesn’t require abdominal incisions, it does require the use of a surgical instrument– the hysteroscope –and thus should still be considered surgery. You can learn more about hysteroscopy on the American Congress of Obstetricians and Gynecologists website.
Essure placement is also commonly advertised as a “quick in-office procedure” which requires no anesthesia or only local anesthesia, but in practice, many patients find implantation painful enough that they need general anesthesia. A 2007 French study found that over 50% of Essure patients actually do end up receiving anesthesia.
Once the Essure implants are placed, the inflammatory PET fibers from the device’s inner coils irritate any fallopian tube tissue that comes in contact with them, causing the immune system to respond by creating scar tissue in the area. Over months, enough scar tissue accumulates to completely block entry to the fallopian tubes, inhibiting fertilization by making it impossible for sperm to pass through to reach the eggs.
For about 3 months after implantation, Essure patients must use a second method of contraception to avoid unplanned pregnancy while the scar tissue barrier is still developing. Then, patients are urged to come in for a follow-up appointment to check whether or not the procedure went as expected.
The standard confirmation test is a particular type of hysterosalpingogram (HSG), which is performed by injecting a special dye into the fallopian tubes through the cervix and observing the movement of the dye by taking x-rays. If the flow of the dye stops at the location where the Essure implants were placed, then the procedure is judged to have been successful.
In 2015, the FDA approved the use of transvaginal ultrasound (TVU) as an alternate Essure confirmation test that doesn’t require x-rays. In TVU, the implantation site is visualized using sound waves from a vaginal probe.
Since Essure needs to be implanted in the body, it belongs in the FDA’s highest risk category for medical devices – Class III. Normally, manufacturers submitting applications for Class III devices are required to complete extensive safety testing as well as rigorous clinical trials that clearly demonstrate a reasonable level of safety before the FDA will even consider approving the device for market release.
However, because Essure was presented as a particularly promising, unique new method of permanent birth control, the FDA decided to grant an “Investigational Device Exemption.” This exemption is meant to speed up the approval process for devices thought to be potential “breakthrough” treatments, and lowers the usual strict application standards by permitting the use of weak data from uncontrolled, non-randomized studies to help demonstrate safety.
In 2002, after evaluating clinical trials and other safety data provided on Essure, an FDA panel voted to allow Essure to be released on the market. But instead of granting outright approval for the device, the FDA gave a “Conditional Pre-Market Approval” (CPMA), allowing the manufacturer to market Essure as long as a list of stringent conditions were met.
Along with typical CPMA demands like following standard manufacturing practices, the FDA required the execution of two postmarket studies, in order to shed more light on safety issues that the agency was particularly concerned about:
Though this post-approval research was reportedly completed by the 5-year follow-up deadline, the scientific integrity of the design and execution of the studies have been called into question by researchers and healthcare advocates.
According to critics, these manufacturer-sponsored studies, along with the early clinical trials submitted in support of Essure’s FDA approval, were heavily biased. Clinical trial participants have accused Bayer / Conceptus, Inc. of manipulating patients during questionnaires and even physically altering survey answers and medical records.
FDA representatives say that although they have seen “occasional modifications” to clinical surveys, they haven’t yet seen substantial evidence for claims of “systematic or intentional” tampering, and the matter is still under investigation. In any case, the near-perfect patient satisfaction ratings and extremely low complication rates boasted by the early clinical trials don’t seem to match up with postmarket feedback from Essure patients, nor with more recent studies on Essure safety and efficacy.
So far, over 10,000 adverse Essure reports have been submitted to the FDA through the agency’s Medwatch program. From an analysis of 4,150 reports submitted between October 2013 and June 2015 – a period of under two years – the FDA compiled a list of frequently-cited side effects and complications:
Though these side effects can vary widely in severity and number among individual patients, many of them prove extremely disruptive to patient quality of life, rendering some patients unfit to work or care for their families due to the debilitating effects of overwhelming pain, fatigue, social anxiety and discomfort. Other patients may suffer long term or permanent health problems from internal damage believed to result from misplaced, malfunctioning, migrated or broken Essure implants.
Until fairly recently, women suffering from Essure side effects had virtually no one to turn to for help. For most of Essure’s time on the market, even physicians and other medical professionals were unfamiliar with or unaware of the device’s risks, which critics say were considerably downplayed or concealed during early clinical trials. Because of this, victims’ complaints of adverse Essure symptoms were often met with disbelief or puzzlement from doctors.
So when New York resident Angie Firmalino banded together with fellow Essure victims to create the Essure Problems Facebook community in 2011, with the simple aim of educating her family and friends about the potentially serious risks of the device, women flocked to the group for much-needed assistance, support and information.
Since then, Essure Problems’ membership steadily rose to over 30,000, and through the heroic efforts of its founding members, administrators, and other supporters, the group has evolved into a powerful grassroots movement to defend the public from Essure’s risks. Essure Problems members are constantly working to get their message heard, often traveling to important medical press conferences, meetings, and patient rights rallies at their own expense.
After receiving such a high volume of adverse reports on Essure, as well as a citizen’s petition organized by Essure Problems that called for a ban on the device, the FDA realized the need to address the public’s safety concerns by reassessing Essure’s risk-benefit profile.
On September 24, 2015, the FDA held a comprehensive panel meeting with the Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee, as part of an in-depth Essure safety investigation. This panel meeting consisted of an Essure background presentation, an Open Hearing in which audience members were invited to speak, comment and ask questions, and a concluding discussion with final recommendations from the panel. Major topics explored included:
Panel members were particularly concerned about the last point, with some experts asserting that if randomized, controlled studies — the “gold standard” in clinical research — had been conducted for Essure, then the medical community could have identified the device’s serious side effects years earlier, potentially sparing thousands of women from suffering complications.
After reviewing the panel meeting, the FDA also evaluated relevant medical literature focusing on Essure and alternative sterilization methods, thousands of adverse event reports submitted over the years, and comments from a public docket.
The agency concluded its investigation by announcing plans to add its strictest alert, the “black box” warning, to Essure product labeling along with a “Patient Decision Checklist” designed to properly inform prospective patients of risks. The FDA released its proposed wording for these items on its official website in the form of a “draft guidance” and requested feedback from the public.
<h2=>Victims Filing Essure Lawsuits Despite Legal Hurdles
Essure’s apparently debilitating effect on health and quality of life can cause patients considerable pain and suffering, often resulting in lost wages as well as putting strain on family relationships and social life.
Some victims are fighting back with legal action against Bayer, claiming that their injuries and hardships could have been easily avoided if the manufacturer had only warned doctors and patients of Essure’s alleged risks.
Unfortunately, Essure plaintiffs seeking rightful compensation for harm inflicted on them currently face an uphill battle due to Essure’s status as an FDA-approved device. The FDA’s authority as a federal agency means that its stamp of approval affords Bayer legal protection from product liability requirements, many of which are based on state laws. Thus, most product liability arguments that victims could make to justify the need for financial relief may be vulnerable to dismissal by “federal preemption.”
But five women from Pennsylvania, undaunted by these challenges, filed the first Essure sterilization lawsuits in December 2014 and January 2015. Although Bayer attempted to have these cases completely dismissed by the court, Judge John R. Padova ultimately allowed the plaintiffs to proceed after reviewing their claims on March 23, 2016 — an encouraging development for current and future Essure plaintiffs.
Essure survivors and their supporters are also pushing for a complete ban of Essure, arguing that the device is “unreasonably dangerous” and unfit to be marketed to the public. A retraction of FDA approval for Essure would also make Essure litigation a lot simpler and more straightforward.
Essure Problems activists have long been reaching out to legislators about these issues. Now, several members of Congress are proposing new bills designed to protect women and their families from Essure and other potentially dangerous medical devices, and to help support the legal rights of injured victims.
This proposed bill calls for a retraction of Essure’s Pre-Market Approval. It was first introduced to the House of Representatives on November 11, 2015 by its primary sponsor, Congressman Mike Fitzpatrick, the Representative for Pennsylvania’s 8th congressional district.
The text of the bill, which can be read on the official U.S. Congress website, states that enactment of the E-Free Act will require the FDA Commissioner to withdraw approval for Essure no later than 60 days after the bill passes.
Right now, the E-Free Act is still at an early stage in the legislative process. After its introduction, it was referred to the Subcommittee on Health and awaits further action. However, the bill, which was originally co-sponsored by 3 additional Representatives, has rapidly gained support, with several more Representatives later signing on as co-sponsors. It’s also already received hundreds of support letters from the American public.
This bill was named after Ariel Grace, an “E-baby” unexpectedly conceived while her mother, Kristiana Burrell, a nurse from North Carolina, was implanted with Essure. According to Burrell’s obstetrician, an Essure coil had ruptured Burrell’s amniotic sac, inducing labor only about 7 months into the pregnancy and leading to Ariel’s Grace’s stillborn death.
Ariel Grace’s Law is also sponsored by Mike Fitzpatrick, along with Representative Louise Slaughter of New York‘s 25th congressional district. First introduced on June 8, 2016 — the one-year anniversary of Ariel Grace’s death — the bill is a proposed update to the Medical Device Amendments of 1976 that would nullify federal preemption of legal claims regarding FDA-approved medical devices. If the bill passes, Bayer would no longer be shielded from state-law-based product liability, removing a significant hurdle to the Essure litigation.
Also introduced on June 8, 2016, by Representatives Mike Fitzpatrick and Louise Slaughter, the Medical Device Guardians Act of 2016 is a proposed amendment to the Food, Drug, and Cosmetic Act which would serve to make adverse event reporting mandatory for physicians.
Current FDA regulations only require manufacturers and hospitals to report any and all adverse events observed, but reporting is optional for doctors and patients. Reps. Fitzpatrick and Slaughter, along with the Act’s supporters, believe that doctors should also be expected to send in their observations of adverse events. After all, with their extensive knowledge and experience as medical professionals, physicians would likely be among the first to notice any medical device “red flags” and could greatly help the FDA identify previously undetected health risks.
The text of the bill simply expands the class of “device user facilities” required to report adverse events to include doctor’s offices, in addition to facilities that were already included (hospitals, nursing homes, and both outpatient and mobile surgical treatment centers). Just as before, those who report adverse events are shielded from potential liability in order to encourage truthful reporting. This means that an adverse event report submitted to the FDA cannot be used as evidence in a civil lawsuit against a doctor or other report writer.
After the first 5 Essure lawsuits were filed in Pennsylvania, more and more women have followed suit with their own legal claims. Dozens of lawsuits representing over 1,000 injured Essure patients are now pending in state courts across the country.
Indeed, as the Essure controversy gains visibility, with both traditional and social media rapidly spreading the word of new developments in Essure FDA actions, proposed legislation, and pending lawsuits, the legal community expects that the number of Essure lawsuits will only continue to rise. If you think you may have grounds for a lawsuit against Essure, our New York defective device lawyers can help you learn more about your legal options in a free consultation.
Continue reading: Abilify Lawsuits Now Being Filed In New York