Homeopathic teething products, which many parents use to ease the discomfort of teething babies, may cause serious side effects. Teething tablets and gels manufactured by Hyland’s Homeopathic, Orajel and CVS have been accused of causing seizures, respiratory issues and other worrying symptoms in infants. At least 10 child deaths have already been linked to the products, which contain trace amounts of an extremely-toxic plant called belladonna.
To learn more about child injuries, see: https://banvillelaw.com/personal-injury/child-injury/
Over the last five years, the US Food & Drug Administration has received more than 400 reports of severe side effects in children. Many of these reports have been consistent with symptoms of belladonna poisoning. In response, the FDA has urged parents to stop using homeopathic teething products immediately. Caregivers are advised to throw out any remaining supply.
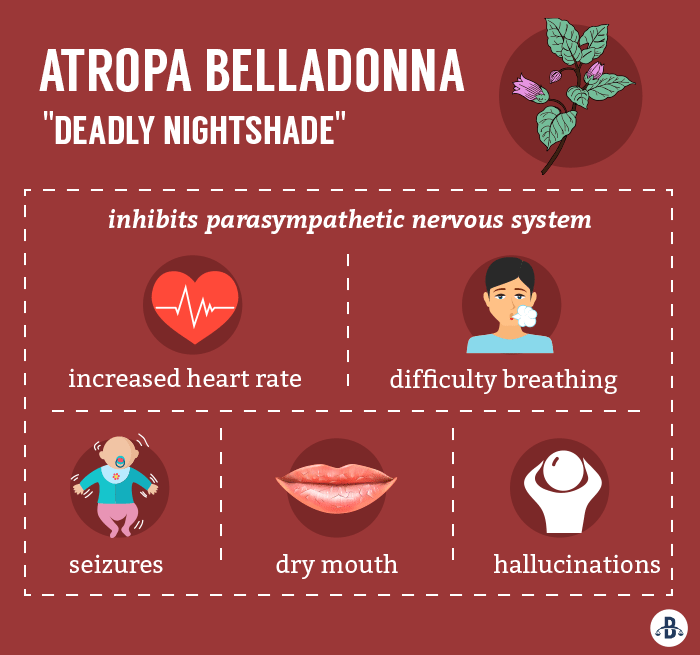
Dry mouth and hallucinations have also been reported in children suffering from belladonna toxicity.

In the wake of FDA warnings, CVS and Walgreens, two of the nation’s largest pharmacy chains, have decided to remove homeopathic teething tablets and gels from their stores. Although Hyland’s products have received the most press, the FDA has cautioned parents to avoid all teething tablets and gels formulated on homeopathic principles, including:
Hyland, one of the country’s largest homeopathic companies, has voluntarily discontinued distribution of its teething products. But the company has so far resisted the idea of a recall, and continues to stand behind the safety of its homeopathic teething tablets and gels. At the time of this writing, the FDA continues to investigate adverse event reports. The agency is conducting laboratory tests of sample teething products, with more information to come.
Belladonna, or “deadly nightshade,” has been used for centuries as both a medicine and poison. The plant’s leaves and berries are infused with a variety of potent toxic chemicals, including atropine and scopolamine, which can shut down nervous system functions like digestion and respiration. While these chemicals have noted medicinal benefits, their effects are highly unpredictable. High dosages can lead to severe side effects and death.
It should be obvious that the manufacturers of homeopathic products, especially ones containing belladonna, have a duty to carefully control the amount of this deadly substance that reaches consumers. However, there is strong evidence that at least one homeopathic manufacturer has not upheld this duty in the past.
Hyland’s Homeopathic came under federal investigation in 2010, after the FDA received multiple reports of children experiencing severe side effects consistent with belladonna poisoning. After tracing the injuries back to Hyland’s Teething Tablets, government health regulators discovered “inconsistent amounts of belladonna” in the company’s products.
Inspections of the manufacturer’s production facility found evidence of “substandard control of the manufacturing operation.” After consulting federal investigators, Hyland decided to issue a voluntary recall for the teething tablets. The product was “reformulated,” according to the company, returning to store shelves in 2011.
Five years after Hyland’s Teething Tablets and Gels reappeared in stores, the FDA is again warning parents and caregivers to discontinue use of the products. At least 400 injuries and 10 deaths have been reported since Hyland “reformulated” its teething products. According to FDA spokesperson Lyndsay Meyer, the side effect reports currently under review “are similar to those observed” five years ago.
While homeopathic products are regulated as medications by the US Food & Drug Administration, they do not undergo rigorous safety and efficacy testing like other drugs. In fact, homeopathic products were explicitly exempted from certain requirements of the Food, Drug & Cosmetics Act – in large part because the Act’s author, New York Senator Royal S. Copeland, was himself a homeopath.
These exemptions are still active. Today, homeopathic manufacturers are allowed to market and sell products intended to treat “self-limiting” medical conditions over-the-counter – without undergoing the FDA’s strict safety testing.
While FDA officials don’t test homeopathic remedies for safety or efficacy, the agency does regulate branding and marketing claims. Labeling appears to be an issue for some homeopathic manufacturers. Hyland’s Homeopathic, for example, has been cited in the past for misbranding its products, including Teething Tablets and Gels, in violation of federal law. The company has also faced a number of class-action lawsuits, filed by consumers over allegations of misrepresenting the effectiveness of homeopathic products.
Homeopathy is not an “ancient” alternative medical system. In fact, its principles were first formulated in 1796 by the German physician Samuel Hahnemann. The two most important principles defy well-established laws in both chemistry and physics:
In short, homeopaths seek out poisonous herbs and minerals, substances that mimic symptoms of an illness, then dilute the toxins in water. After successive dilutions, there is often no active ingredient left in the solution. To explain how water could still provide therapeutic benefits, homeopaths often cite a third principle: “water memory.” According to this theory, which was only introduced to the field of homeopathy in the 1980s, even highly-diluted solutions retain a “memory” of the active ingredient.
Like the principles of “like cures link” and “minimum dose,” the idea that water has a “memory” is scientifically unfounded. In large clinical studies, homeopathic remedies have shown to provide no more than a placebo effect. The “medications” are no more effective than sugar tablets. In addition, the assumption that all homeopathic remedies are so diluted that side effects become impossible isn’t accurate. According to the National Institutes of Health, “some products labeled as homeopathic can contain substantial amounts of active ingredients and therefore could cause side effects and drug interactions.”
With serious side effects reported across the country, our attorneys believe that some families may be eligible to file product liability lawsuits against the manufacturers of homeopathic teething tablets and gels. If your child suffered symptoms of belladonna poisoning, including seizures or difficulty breathing, we can help.
Contact our experienced lawyers today for a free consultation. Eligible families may be able to pursue financial compensation, money to cover medical expenses and emotional trauma. Learn more about your legal options – at no charge and no obligation – now.
Continue reading: Jury Finds Driver Under The Influence Responsible For Accident That Killed A Child